

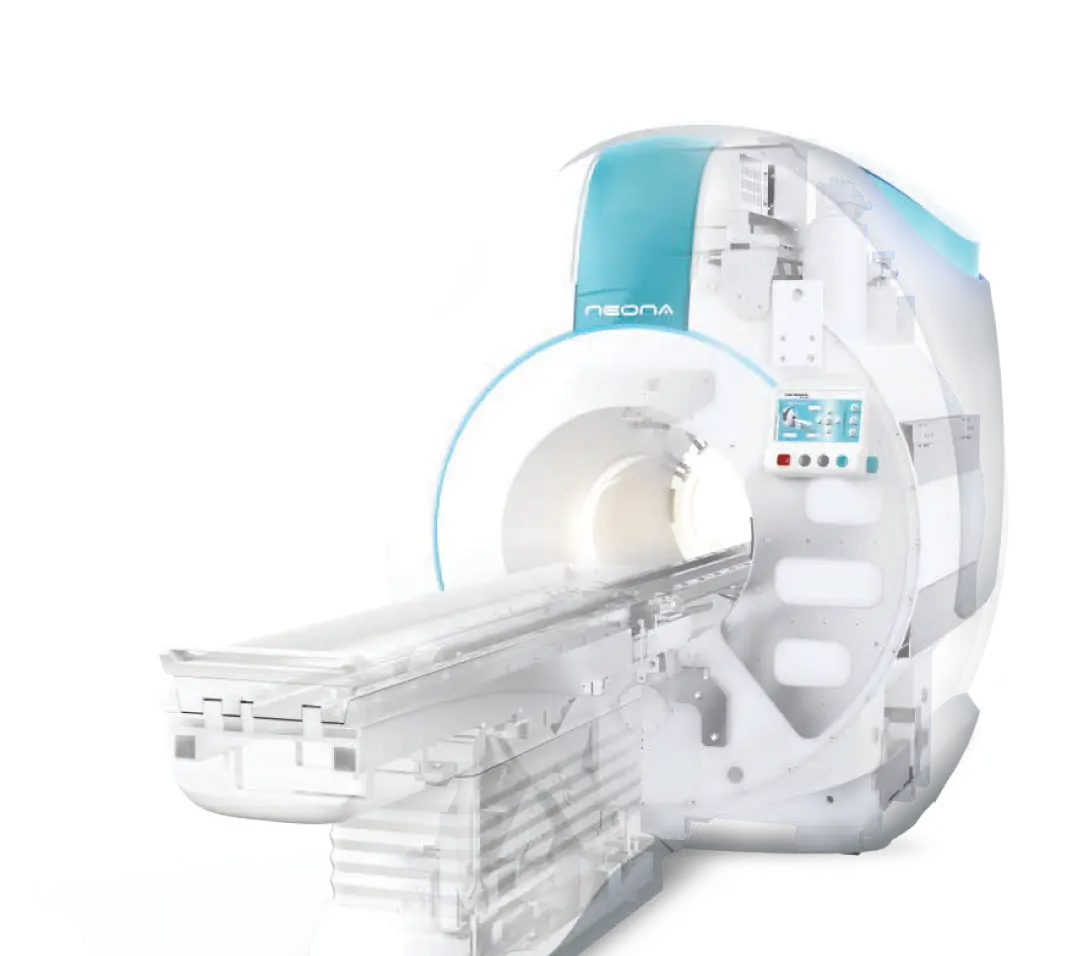
The NEONA has most updated cryo-free magnet which does not require quench pipe. It also has compact footprint, light-weighted magnet and is specifically designed for installation within Neonatal Intensive Care Unit (NICU). It has incorporated with fast scanning protocols with reduced acoustic noise. The strong gradient ensures the high image quality in terms of high spatial resolution and signal-to-noise ratio under short acquisition time.
US FDA 510(k) is cleared.

Features Overview
The NEONA MRI was designed from the onset to address very special needs including the Neonates that reside in the neonatal intensive-care unit (NICU) of large Medical Facilities. The design plan involved addressing two major problem areas that are particularly unique for this segment of critically ill infants.
The first issue required a compact design for the MRI system that would permit its location within or very near the NICU area. This co-location concept for patients and equipment minimizes patient transport issues and affords lower risk access to the MR technology.
The other issue involves the use of an MR compatible Neonatal Incubator during the actual MRI exam. The MR-compatible incubator serves the purpose of monitoring and stabilizing the patient's physiological and environmental needs as well as including the MR Coil components themselves. The incubator design requirements - both structural and functional are quite specific and are best addressed by incorporating the Incubator as an integrated sub-system of the MRI system itself.
Collectively - the broad scope of unique features of the NEONA MRI differentiates it significantly from other traditional general purpose MRI systems. We believe this additional investment of design effort addresses more Neonate categories and will better serve as the development platform for further advances in the near and long term. Only in this manner can we expect to advance and improve MRI utility with this unique patient population.
Features & Benefits

1.5T Cryo-free
Superconducting Magnet

No Quench Pipe
required

Safety
Maintain a safe Specific
Absorption Rate(SAR)

Improve
image quality for diagnostic
purpose on neonates

Light weight & Compact
for installation on upper floors

Ultra-small FOV
Design of RF receiver coils

Specialized pulse sequences
for neonatal scans

Noise reduction
Optimize gradient acoustic
noise for best patient comfort

Award in International Exhibition of Inventions of Geneva
Time Medical has participated in the world leading IP convention. Time Medical exhibited with the World’s First Neonatal MRI System - NEONA and won the Prix de l’Etat de Genève award which is the highest award among the medical class.
NEONA, the 1 superconductive neonatal MRI in the world
Compared with regular MRI, NEONA is a much safer diagnostic system for neonates with a substantial reduction of RF power Specific Absorption Rate (SAR) and as well as acoustic noise. With the Neonate tailor-made system, software and pulse sequence, NEONA provides the fast and precision neonate imaging service. The system is more compact and lighter, it can be easily installed inside/nearby the Neonatal Intensive Care Unit (NICU), reducing the risk in transporting the prenature neonates in hospital.
Product Gallery

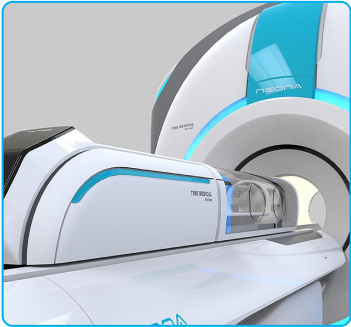
NEONA- The World’s
First Neonatal MRI System
FAQs
NEONA is the world’s first MRI system specifically designed for imaging neonates and infants. It offers safe, high-resolution scans within the NICU environment.
Unlike traditional MRI machines, NEONA is compact, quiet, and integrates an MR-compatible incubator to ensure safety and stability for newborns during imaging.
NEONA is built with a 1.5 Tesla superconducting magnet, optimised for neonatal imaging to provide high-quality diagnostic images.
Yes, its compact and lightweight design allows it to be installed in or near the NICU, reducing the need to move fragile newborns for scanning.
Yes, NEONA has received FDA 510(k) clearance and complies with international medical device standards for clinical use.
Yes, NEONA reduces scan time by up to 50%, acoustic noise by up to 80%, and RF power exposure by 75% compared to traditional MRI systems.


